"Lilly is supportive of the decision by the independent Data Safety Monitoring Board (DSMB) to cautiously ensure the safety of the patients participating in this study," said the company.
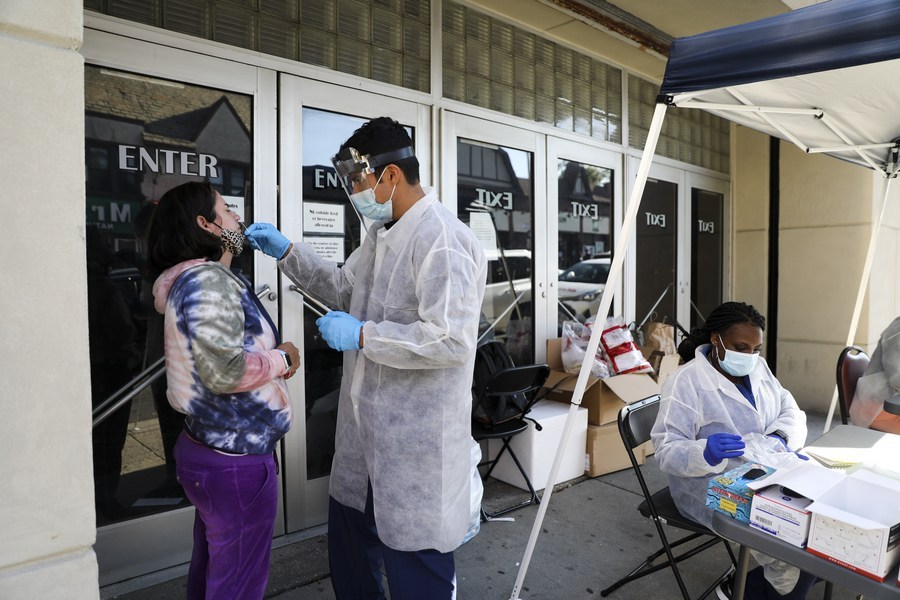
U.S. drugmaker Eli Lilly said Tuesday it has paused its trial of a combination antibody treatment for COVID-19 for safety reasons.
The company said the trial's Data Safety Monitoring Board (DSMB), an independent group of medical experts who monitor clinical trials, recommended the pause.
"The trial, evaluating Lilly's investigational neutralizing antibody as a treatment for COVID-19 in hospitalized patients, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH)," a Lilly spokesperson said in a statement.
"Lilly is supportive of the decision by the independent DSMB to cautiously ensure the safety of the patients participating in this study," said the statement.
Neither Eli Lilly nor the NIAID, which is sponsoring the trial, have described the safety issue that prompted the decision to pause the study.
(Source: Xinhua News Agency)



















Comment